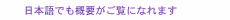|
|||
|
|
|||
|
Special Feature on Technical Solutions to Real-world Problems Vol. 6, No. 11, pp. 35–37, Nov. 2008. https://doi.org/10.53829/ntr200811sf3 Anti-corrosion Measures for Cable Bearers and Other Iron Fittings in ManholesAbstractThis article introduces countermeasures to corrosion caused by corrosive ions in accumulated water and high humidity, which can be frequently seen in cable bearers and other iron fittings inside manholes that support underground communication facilities.
1. Corrosion of cable bearers in manholesThe 1960s and 1970s saw a massive deployment of access-system and trunk-system facilities in Japan. For many of these facilities, it has been more than 30 years since they were first installed and the time for upgrading them is fast approaching. In particular, iron fittings installed in manholes used for communication purposes are often exposed to humid conditions that frequently result in their deterioration due to corrosion. For example, cable bearers mounted on the wall of a manhole, which are made of steel plates only 1.6 mm thick, may break under the weight of a cable after being corroded (Fig. 1).
2. Mechanism of metallic corrosionWater and oxygen can be regarded as the causes of metallic corrosion. Corrosion can be promoted when these two factors interact with metal in just the right way. Moreover, chloride ions and an acidic atmosphere promote metallic corrosion, so corrosion is very severe in coastal areas and hot-spring regions. Corrosion can also be accelerated by microorganisms, such as sulfate-reducing bacteria. Sulfates can be provided by an intrusion of fertilizer or sewage, for example, and they can then be consumed by bacteria as nutrition, resulting in the creation of an acidic atmosphere that can promote metallic corrosion. Taking a more detailed look at the corrosion of iron, we see that it is electrochemical in nature, as shown in Fig. 2. This illustration shows how iron (Fe) can dissolve into Fe2+ and become ferric oxyhydroxide (FeOOH) or rust on the surface of iron. All corrosion occurs as a result of such electrochemical phenomena.
3. Countermeasures to metallic corrosionThere are two countermeasures to metallic corrosion: (1) blocking off water and oxygen and (2) protecting metals by electrochemical methods. A popular method of blocking water and oxygen is to apply a coating to metal surfaces. This has been frequently used in construction partly for aesthetic purposes; however, it has not been widely used for manholes. The method that has been commonly used for manholes as well as for aerial fittings is hot-dip galvanization, which is generally called zinc plating or just simply plating. This makes good use of the anti-corrosion effect of zinc itself as well as the electrochemical property whereby the zinc corrodes before the iron. However, if the environment within a manhole is very severe, then even the use of plating will not prevent the corrosion described above. 4. Anti-corrosion measures for a manhole environmentHere, we introduce two of the products developed by the Technical Assistance & Support Center at NTT East for the corrosive environment within manholes. (1) Oxygen and water blocking method Using products with an organic lining is a typical method supporting the installation of new facilities or the upgrading of existing ones. For example, a cable bearer already given zinc plating can be lined with thermoplastic polyester, resulting in a structure with two layers of protection against corrosion. Such products have been used in stay-wire anchors since 1988 and in the underground and ground-level portions of eco steel telephone poles (LL steel telephone poles, etc.) since 2003. Products for all the iron fittings in a manhole are being prepared, beginning with cable bearers, and will include metal ladders (Fig. 3).
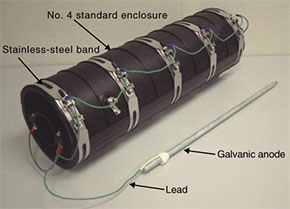
If rust has already formed and we want to halt its progress without upgrading, then environment-friendly tallow-based rust-penetrating, anti-rust paint (Eureka) can be applied. This is an anti-corrosion agent based on the fat of sheep's wool as the main raw material. It can be applied manually in aerosol or cream form to protect the surface of the target object and it penetrates the rust to produce an anti-corrosion effect. (2) Electrochemical method A galvanic anode system can be used as an electrochemical method. This system protects the iron fitting from corrosion by electrically coupling it with a metal that will corrode before iron. This has the effect of halting the progress of corrosion, which means that the system can be used for preventive maintenance in addition to preventing rust from progressing in existing iron fittings. Galvanic anodes made of magnesium alloy have been prepared for use with iron fittings within a manhole. There is a product that provides common support for iron fittings such as cable bearers and braces and one for use with pooling bolts (Fig. 4). Moreover, an iron-based galvanic anode has been prepared to prevent the rupture of stainless-steel bands on a standard closure installed in manholes at risk of taking in seawater (Fig. 5).
5. ConclusionThe coming of the optical communications era will make it increasingly important to protect and maintain cables. There have been reports of excessive force being applied to connection points as a result of cable bearer corrosion and this sometimes leads to failure. Cable bearers and other iron fittings with exceptional durability should be used for upgrades made to rectify corrosion-related deterioration. It is also important to take preventive-maintenance measures based on galvanic anodes. |
|||