|
|||||||||||
|
|
|||||||||||
|
Special Feature: NTT Group R&D for Reducing Environmental Load Vol. 7, No. 10, pp. 26–30, Oct. 2009. https://doi.org/10.53829/ntr200910sf5 Sensor Element for Indoor Formaldehyde MeasurementAbstractWe have developed a passive sensor element for detecting formaldehyde. It works by using the chemical reaction between formaldehyde and both β-diketone and ammonium ions on a porous glass surface. Lutidine derivative (yellow dye) is formed as a reaction product, and the absorbance at around 410 nm increases. The formaldehyde concentration is calculated from the change in absorbance of the sample after exposure. Indoor formaldehyde concentrations in the Kanto area of Japan have been determined. Using this sensor element, we have successfully detected ppb levels of formaldehyde.
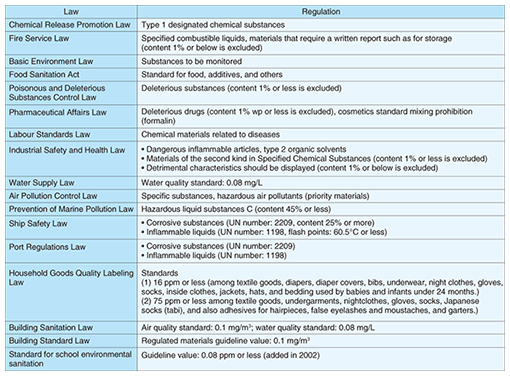
1. IntroductionFormaldehyde (HCHO) is a reactive gas emitted by various off-gassing sources, such as building materials and furniture, and also one of the source materials that cause sick building syndrome. The World Health Organization (WHO) guidelines for residential indoor air quality set the formaldehyde exposure limit at an average value of 80 parts per billion (ppb) for 30 minutes [1]. Formaldehyde is a colorless reactive gas at ordinary temperatures and pressures, and it is an irritant with an unpleasant odor. It dissolves easily in water, and the resulting liquid is called formalin. Outdoors, formaldehyde is readily photooxidized to carbon dioxide in sunlight, and it reacts with air pollutants. Therefore, the half-life of formaldehyde is about 50 minutes in the absence of NO2 gas and about 35 minutes in its presence. Outdoor formaldehyde concentrations are lower than indoor ones. The outdoor emission sources of formaldehyde are mainly the gases emitted by cars and factories. By contrast, the indoor emission sources of formaldehyde are resin products, adhesives, chipboard, fabric, tobacco smoke, and heating devices. Formaldehyde is an important industrial material with a huge annual production volume of over one million tons in Japan and 20 million tons worldwide, and it is used as a synthetic raw material in the production of various compounds including resinoids, chemical reagents, drugs, and medicines. The many Japanese laws regulating formaldehyde and formaldehyde measurement are summarized in Table 1. These regulations show that formaldehyde is used in many fields, and that many people have the potential to be affected by formaldehyde vapor. We therefore believe that it is very important to determine the formaldehyde concentration in the atmosphere and to utilize formaldehyde safely. Our small, lightweight sensor provides an excellent way of monitoring the formaldehyde concentration around us.
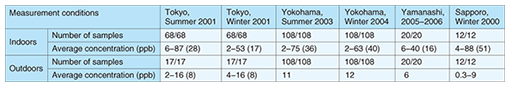
Indoor and outdoor formaldehyde concentrations measured in metropolitan Tokyo, Yokohama [2], Yamanashi prefecture [3], and Sapporo [4] are summarized in Table 2. The indoor formaldehyde concentration is usually 2–6 times higher than the outdoor concentration, and the indoor concentration range is wider than the outdoor range. Since people spend about 80% of their time indoors, the major anthropogenic sources that affect human beings are found in the indoor environment.
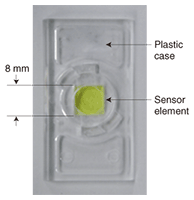
Acute exposure to formaldehyde causes irritation of the eyes and upper airways, and long-term exposure to low levels has been associated with an increased risk of developing respiratory illnesses. In 2004, formaldehyde was classified as a human carcinogen by the International Agency for Research on Cancer. In Japan, formaldehyde was classified as a group II Specified Chemical Substance in 2004. Therefore, a sensor that can monitor the indoor formaldehyde concentrations is needed in order to evaluate human exposure levels. 2. Measurement of formaldehyde levelsOne well-known formaldehyde measurement technique is the DNPH method. Formaldehyde is collected in the form of 2,4-dinitrophenylhydrazones (DNPHs) by drawing air through a DNPH-silica cartridge. The contents of the cartridge are extracted using organic solvent and the eluate is analyzed by high-performance liquid chromatography. Although accurate results are obtained, this analytical method requires many complex procedures and takes several days to produce results. Furthermore, measurements in a closed area may be impossible to perform because the method requires a pumping unit. Three measurement methods have been approved as simple formaldehyde measurement methods by the Japanese Health Ministry, namely a mobile formaldehyde sensor with a 3-minute monitoring time (Biomedia, Japan), a formaldehyde detecting device (Rikenkeiki, Japan), and a formaldehyde analysis kit (Gas-Tech, Japan). However, they all require a device equipped with a pumping unit, and the measurement time is restricted to between 3 and 30 minutes. This makes it impossible to perform measurements in a confined space or to measure the average formaldehyde concentration over a period of 1 day. We have developed a formaldehyde sensor with excellent properties. It is small, easy to use, and highly accurate. Moreover, the measurement period can be set arbitrarily, measurements are possible in confined spaces, and on-site analysis is possible. 3. Developed sensor elementThe developed sensor element housed in a plastic case is shown in Fig. 1. We used a porous glass as a substrate, which we impregnated with both β-dike tone and ammonium ions. Lutidine derivative (yellow dye) is formed as a reaction product and the absorbance of the sensor element increases at around 410 nm. We found a linear relationship between the amount of lutidine derivative and the product of the formaldehyde concentration and the exposure time. We can calculate the amount of lutidine derivative from the change in the sample’s absorbance, so we can calculate the formaldehyde concentration in the same way. We also found that we could set the measurement period arbitrarily because the lutidine derivative was stable under weak light conditions (e.g., a few hundred lux).
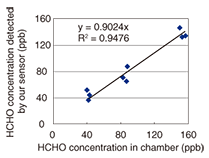
We have also developed a device for measuring the sensor element’s absorbance. In this device, a light emitting diode and a photodiode are used as a light source and a detector, respectively. We confirmed that we could measure the sensor element’s absorbance with high accuracy without the need for a large analytical instrument. This makes possible on-site analysis of formaldehyde concentration from the absorbance change measured using our small device. We can also determine the approximate formaldehyde concentration visually by checking the sensor’s color against a color chart. Moreover, we can convert a captured sensor element image into a formaldehyde concentration. We determined that the error in the concentration measurement resulting from the image conversion was ±10 ppb. The sensor element shown in Fig. 1 is small enough to be positioned almost anywhere in a house. It can measure formaldehyde concentrations in confined spaces, such as drawers, because the sensor element can function without an active sampling device. The results of a chamber test are shown in Fig. 2. This test was carried out at the Chemicals Evaluation and Research Institute (CERI), Japan, which is an independent performance evaluation organization. We prepared three different concentrations of formaldehyde (40, 80, and 160 ppb, which are 50%, 100%, and 200% of the WHO guideline value, respectively). Gas at each concentration was introduced into a chamber containing a sensor element. The exposure period was 30 minutes, which corresponds to the WHO guidelines, and the temperature and humidity were 25ºC and 50%RH, respectively. The exposure experiment was performed three times for each concentration. We obtained a coefficient of variation of 17% and a correlation coefficient of 0.94. These results revealed that the developed sensor element can measure formaldehyde concentrations easily and highly accurately without the need for a pumping unit.
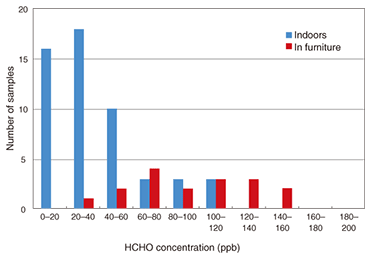
4. Measurements in residential houses and furnitureWe contacted volunteers who wanted to know the formaldehyde concentrations in their houses or furniture and asked them to measure them using our sensor element. There were 25 volunteers, who took 54 samples. An exposure time of 6–24 hours was selected arbitrarily, with the average exposure time being 8 hours. Measurements were performed on an arbitrarily selected day between October 1 and 30, 2007. We provided the volunteers with sensor elements housed in plastic cases for ease of handling. Each volunteer exposed the sensor element for an arbitrarily selected period of 6–24 hours at a location of their own choice where they wished to know the formaldehyde concentration. Each volunteer then placed the exposed sensor element in an aluminum-coated plastic bag, sealed it airtight, and returned it to us. We measured the spectrum of each sensor element and calculated the formaldehyde concentration from the absorbance change at 414 nm. The results of a formaldehyde concentration histogram analysis of residential houses and furniture are shown in Fig. 3. There is a concentration peak for residential houses at 20–40 ppb, and most values are less than 80 ppb (WHO guideline value). Relatively high concentrations were measured in both new houses (0–2 years old) and in houses built before the revision of the Building Standards Law (over 2 years old), which all lack a ventilation system. The formaldehyde concentrations in furniture ranged from 20 to 160 ppb, which are relatively high concentrations. Drawers contain many formaldehyde sources including chipboard, adhesives, and clothes, and we expected high concentrations.
5. Concluding remarksWe successfully detected ppb levels of formaldehyde using our developed sensor for arbitrarily selected exposure periods and locations. The sensor element is affected by few other gases (interference gases) because it uses a chemical reaction to detect formaldehyde. In particular, acetaldehyde, which belongs to the aldehyde group, caused little interference with the sensor element, indicating that our measurement technique provides good selectivity. We intend to continue research and development to make a range of sensor elements that can respond to various different environmental chemicals with good selectivity and high accuracy because there are many chemicals in our environment. We believe that this chemical-reaction-based sensor technology will lead to technology for improving the environment because the two technologies have many common factors. References
|
|||||||||||