|
|||||||||||||||||||||||
|
|
|||||||||||||||||||||||
|
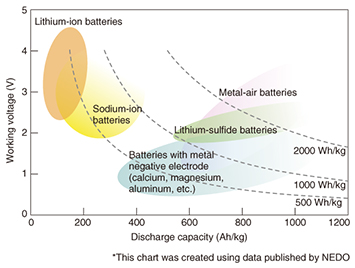
Feature Articles: Reducing the Environmental Burden of ICT Services Vol. 13, No. 3, pp. 23–29, Mar. 2015. https://doi.org/10.53829/ntr201503fa4 Materials Technology for the Production and Storage of EnergyAbstractSlowing the progression of global warming is an urgent task, and the NTT Group recognizes the need to reduce the carbon dioxide emissions that come with our corporate activities. Another important requirement from the viewpoint of the business continuity plan is preparing backup systems in the event of power failures that result from large-scale disasters. For these reasons, NTT Energy and Environment Systems Laboratories has been researching and developing materials for producing electrical power and fuels from natural energy sources such as sunlight and materials for storing natural energy to serve as a backup source of power during power outages. We describe here clean energy systems (artificial photosynthesis, algae, and thermal power generation), which can be used as energy producing materials, and lithium-air rechargeable batteries, which can be used as an energy storage material. Keywords: rechargeable batteries, artificial photosynthesis, algae and thermal power generation 1. IntroductionThere is renewed recognition of the need to achieve a sustainable society that does not burden society or the environment. Particularly in the aftermath of the massive destruction caused by the earthquake that occurred in eastern Japan in 2011, there is heightened interest in technology for energy-producing materials that make use of renewable energy and unused energy sources, and technology for energy-storing materials such as storage batteries (rechargeable batteries). While much research and development (R&D) is being done in the fields of materials for energy production and storage, and new technologies are being introduced, it will probably take some time to develop a market for such technology. This article describes R&D on next-generation materials technology that will contribute to achieving energy production and storage systems. 2. Energy-storing materials: trends and R&D2.1 Expectations for high-performance rechargeable batteriesThere is an essential need to install backup electrical power systems to maintain stable communication services during disasters and the ensuing power outages. Many communication facilities are equipped with motor-driven electrical power generators and lead-acid batteries as backup power supply systems. To provide sufficient power for large telecom facilities and datacenters, however, a large amount of stored fuel or as much as an entire floor of dedicated lead-acid storage batteries is required. These circumstances have created a need for a new type of rechargeable battery that has a higher energy density to provide the same amount of power with less volume and weight than lead-acid storage cells. One approach to solving this problem is to scale-up the lithium-ion batteries that were initially developed in a smaller form to power mobile phones [1]. Ultimately, however, there is no doubt that a new type of rechargeable battery that has even higher energy density than lithium-ion batteries will be required in the future. 2.2 Lithium-air rechargeable batteries as a new form of rechargeable batteryA mapping of the performance of new types of rechargeable batteries, including lithium-ion batteries, provided by the New Energy and Industrial Technology Development Organization (NEDO) [2] is presented in Fig. 1. We can see from the figure that all the new types of rechargeable batteries have higher energy density than lithium-ion batteries. Of those new types, metal-air batteries have the highest energy density.
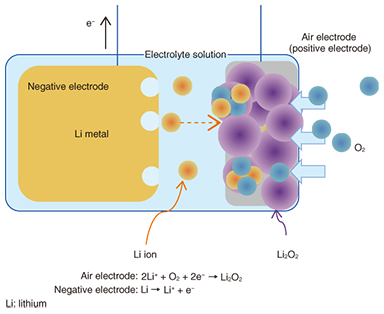
The structure and working mechanism of a lithium-air rechargeable battery is illustrated in Fig. 2 as an example of a metal-air battery. Conventional batteries include the active materials of the positive and negative electrodes within the battery. In contrast, oxygen, which is an active material for the positive (air) electrode of the metal-air battery, is provided from the surrounding air. Because most of the metal-air battery is made up of the metal negative electrode, this type of battery features a very high energy density and a long discharge time. The negative-electrode materials used in metal-air batteries include zinc (Zn), iron (Fe), aluminum (Al), lithium (Li), and other metals. The battery voltage and energy density differ according to the metal that is used, and the lithium-air rechargeable batteries provide a high voltage on the 3-V level as well as a higher energy density than batteries using other metals.
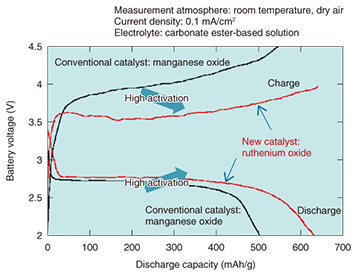
NTT Energy and Environment Systems Laboratories has taken these advantages into account and is proceeding with research on lithium-air rechargeable batteries, which theoretically have a much higher energy density than lithium-ion batteries, with a view to using them in the future as an energy buffer for natural energy sources as well as for backup power supply systems. 2.3 Issues facing lithium-air rechargeable batteriesThe main thrust in research on lithium-air rechargeable batteries began in the first decade of this century. Manufacturers and various research organizations are currently proceeding with basic research to verify the practicality of this technology, and that work has shown that lithium-air rechargeable batteries have low charge/discharge cycle performance, meaning that the discharge time after charging is short, and that it is difficult to obtain a large current. Another technical problem that has become clear is the effect of moisture in the air. Various approaches have been taken to solve these problems by developing battery materials, and in particular, NTT Energy and Environment Systems Laboratories has been researching catalysts that can enhance the reaction rate at the air electrode [3–7]. 2.4 Higher charge/discharge performance through development of air electrode catalysts and electrolyte optimizationOur research shows that ruthenium oxide has higher activity than conventional catalysts. The initial charge/discharge curves of lithium-air rechargeable batteries that use a ruthenium oxide catalyst and a conventional manganese oxide catalyst are shown in Fig. 3. We can see that the ruthenium oxide catalyst has a higher discharge voltage and a larger discharge capacity, and that the charge voltage is low and the charge capacity is large. These results indicate that ruthenium oxide has higher catalytic activity than conventional catalysts.
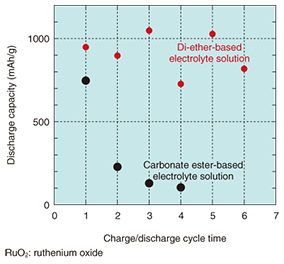
The choice of electrolyte solution is another important factor in improving the cycle performance. A comparison of the charge/discharge performance for a lithium-air rechargeable battery that uses a conventional carbonate ester-based electrolyte and a battery that uses a newly selected di-ether-based electrolyte is shown in Fig. 4. We can see a clear improvement in the cycle performance with the di-ether-based electrolyte.
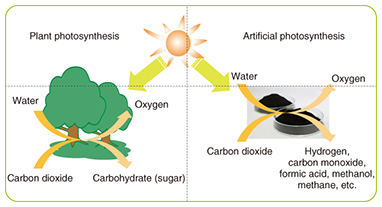
These results indicate that optimization of battery materials is important for improving the charge/discharge cycle and other aspects of battery performance. 2.5 A new future with high-performance rechargeable batteriesIf high-performance rechargeable batteries that have high energy density can be made practical, it will be possible to greatly reduce the size and weight of backup power supplies, which are currently heavy and consume a lot of space. In telecom facilities that dedicate as much as an entire floor of the building to the backup power supply, that would free up a large amount of space that could be used for servers or other equipment. If batteries with sufficiently high cycle performance can be achieved, it would expand the range of applications beyond backup systems. For example, large rechargeable batteries introduced to private residences would allow power to be stored during the night when rates are relatively low, for use during the day. It would also allow homes that have solar panels but do not use much power during the day to store power for use at night. Such peak power cutting or shifting to reduce power consumption can help reduce carbon dioxide emissions. 3. Energy-producing materials: trends and R&DConcerns about the problem of the contribution of carbon dioxide, methane, and other greenhouse gases on global warming, as well as the problem of dwindling fossil fuel resources have been increasing year by year. This has led to an increase in the demand for development of clean energy technology for utilizing renewable and unused energy. In contrast to energy that is derived from petroleum, coal, natural gas, and other fossil fuels, renewable energy exists in the natural world in the form of sunlight, wind, and geothermal heat, for example. Unused energy includes heat contained in rivers and drainage systems and the heat generated by power transformer substations. In particular, these forms of energy are characterized as non-depletable and present everywhere, and they do not emit greenhouse gases or increase the emission of greenhouse gases. Such energy sources therefore have the potential to be a radical solution to the problems mentioned above when used well. We have been focusing efforts on using these forms of energy in the development of green energy technology including green fuels, green power generation, and the capturing of carbon dioxide. We describe here a green fuel based on artificial photosynthesis, the use of microalgae to capture carbon dioxide, and thermoelectric conversion as a means of green power generation. 3.1 Research on artificial photosynthesisArtificial photosynthesis is a process that mimics the photosynthesis of plants by using sunlight to cause a reaction between carbon dioxide and water in order to produce fuels such as hydrogen, carbon monoxide, formic acid, methanol, and methane (Fig. 5). The artificial photosynthesis process uses renewable energy from sunlight (non-fossil fuel) and consumes carbon dioxide, which is a cause of global warming. Currently, though, the efficiency of converting solar energy in the conversion of carbon dioxide to methane and other fuels is very low, and the process is inferior to natural photosynthesis. We are therefore investigating the use of photocatalytic thin films and a way to improve the performance by modifying the thin-film surface with promoters [8]. So far, we have used known photocatalysts for the thin-film, constructed a system for evaluating performance, and confirmed the generation of hydrogen, methane, and carbon monoxide by instigating a reaction between carbon dioxide and water (Fig. 6).
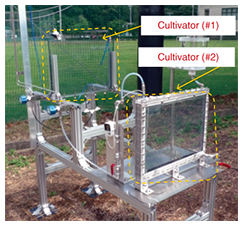
3.2 Research on microalgaeAlgae, especially microalgae, are attracting attention as a fuel resource that does not compete with food sources. Microalgae have a very high rate of reproduction compared to terrestrial plants, as well as a high lipid content and a carbon dioxide capture capability about 10 times higher than a forest. We have been conducting R&D on a system for chemically capturing carbon dioxide using microalgae [9, 10]. We have also been investigating the effects of growth conditions such as the composition of the cultivation medium and temperature, and evaluating the growth rate of algae and the amount of carbon dioxide capture with the objective of optimally controlling the cultivation conditions (Figs. 7 and 8).
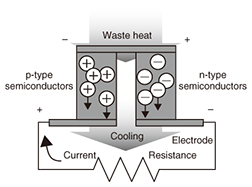
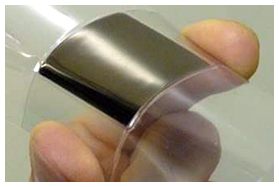
3.3 Research on thermoelectric conversionThermoelectric conversion is a process in which electrical power is generated when there is a temperature gradient within a thermoelectric element. The thermoelectric element comprises p-type and n-type semiconductors that are arranged in a π-type series (Fig. 9). The use of inorganic materials for thermoelectric elements is widely known, but there are problems with their widespread use in general society, as they contain rare or toxic metals, and are hard and heavy. For that reason, we are researching organic thermoelectric conversion materials. Organic materials offer the potential for lightness, workability, and flexibility. They are also less expensive and have less impact on the environment because they contain no rare metals. However, organic materials have lower thermoelectric conversion efficiency than inorganic materials, so there is a need to raise that efficiency. We have therefore been moving forward with the development of practical thermoelectric elements with two objectives: raising the element performance and increasing the element surface area [11]. We have already achieved an element surface area of one square inch (Fig. 10).
4. Future prospectsNatural energy such as sunlight and unused energy such as waste heat can be found everywhere. It is important to collect these forms of energy efficiently and convert them into high-quality energy. By advancing the basic materials research, we aim to dramatically improve the efficiency of such processes. References
|
|||||||||||||||||||||||