|
|||||||||||||||||||||
|
|
|||||||||||||||||||||
|
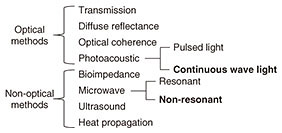
Regular Articles Vol. 17, No. 11, pp. 40–45, Nov. 2019. https://doi.org/10.53829/ntr201911ra1 Noninvasive Glucose Measurement Using Electromagnetic Waves: Photoacoustic Spectroscopy and Dielectric SpectroscopyAbstractNoninvasive glucose measurement without needle pricking is anticipated as a novel medical and healthcare application. We introduce here our research on the use of near-infrared photoacoustic spectroscopy and microwave dielectric spectroscopy for noninvasive glucose measurement using electromagnetic waves. We also present our recent work involving in vivo measurement. Both measurement techniques are based on optical and wireless components and system integration, which have been investigated in telecommunication system development at NTT. Keywords: noninvasive glucose measurement, photoacoustic spectroscopy, dielectric spectroscopy 1. IntroductionThe number of diabetes sufferers has increased greatly throughout the world in recent years [1]. Effective management of blood glucose level for diabetes care relies on having accurate glucose monitors such as self-monitoring blood glucose monitors, continuous glucose monitors, and flash glucose monitors (FGMs). There is a huge demand for noninvasive glucose testing to enable patients to check their own glucose level without the need for reagents or the use of needles to take blood samples. Furthermore, such a testing method could also possibly be used in novel Internet of Things devices for people other than diabetics. They would be able to acquire time series data of changes in their glucose level as effectively as done with conventional wearable devices such as acceleration sensors and heartbeat sensors. A noninvasive testing method would measure individual glucose metabolism and is therefore a potential tool for predicting the risk of diabetes and improving one’s quality of life. Various noninvasive glucose monitors for patients with diabetes mellitus have been studied in the last few decades [2]. The noninvasive concept was first described more than 30 years ago. Nevertheless, most of the current noninvasive technologies are still in their early stages of development. A list of noninvasive methods that have been developed is given in Fig. 1 [3].
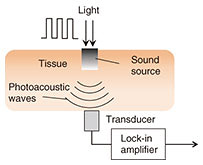
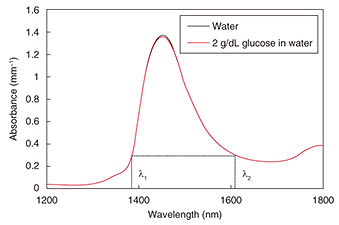
Optical techniques were initially investigated, for example, optical transmission spectroscopy, diffuse reflectance spectroscopy, optical coherence tomography, and photoacoustic spectroscopy (PAS). Non-optical techniques such as bioimpedance spectroscopy, microwave spectroscopy, ultrasound, and heat propagation were investigated later. Some of these techniques have been proven to work in in vivo studies. However, there is still no approved noninvasive testing method. For proof of applicability of such a method, it needs to be tested with a large number of people in actual usage situations, and the measurement instability arising from human physiological characteristics needs to be resolved. We describe here our recent research on noninvasive glucose monitoring, focusing in particular on near-infrared (NIR) PAS and microwave dielectric spectroscopy (DS). These technologies are advantageous in that they enable compact equipment designs that can be applied to wearable devices in the future because they can potentially use commercially available optical and wireless components used for telecommunication systems. We explain the sensing principles and the results of physiological-range glucose detection in in vitro samples using PAS and DS. Finally, we present in vivo results of each method compared with a commercial FGM. 2. PASIn diffused reflectance optical spectroscopy, the repeatability of calibration models can be severely affected by skin conditions because transmitted light is affected by the variations in light scattered in skin and tissue. In contrast, PAS involves direct detection of acoustic waves propagating through tissue without them being scattered. It thus has potentially better tolerance. It has been demonstrated with nanosecond pulsed excitations in the visible, NIR, and mid-infrared regions [3, 4]. We focus here on NIR PAS. We investigated the use of semiconductor lasers of the sort found in optical telecommunications because they make it possible to produce sensors that are both portable and reliable. The concept is shown in Fig. 2 [4]. When tissue is irradiated with two intensity modulated lasers in antiphase, a differential photoacoustic wave is generated. The traveling acoustic waves are detected with a transducer. We used laser lights at wavelengths of 1.38 and 1.61 µm. As shown in Fig. 3, the water absorption is equal at the two wavelengths, but the glucose absorption is slightly different. Therefore, the differential photoacoustic source mainly depends on the glucose concentration.
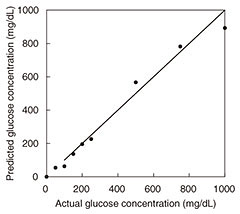
Photoacoustic wave generation involves light-tissue interaction and successive thermoelastic conversion processes. In light-tissue interaction, photons are instantly absorbed by molecules in the tissue and transformed into the kinetic energy of the molecules. Because the laser lights used in biomedical applications are low in power, other photon-molecular interactions such as radiative emission and chemical reactions are negligible. The signal-to-noise ratio (SNR) is determined by system parameters including device performance and signal processing parameters. To achieve a better SNR, the experimentally determined modulation frequency at which the transducer has the highest responsivity is used for each measurement. In addition, because the cell length L corresponds to the n-th acoustic longitudinal resonance mode in closed ends (n = ωL/πν), the frequency also corresponds to the fifth longitudinal mode of the cell cavity [5]. This results in an SNR of more than 30 dB. The results of in vitro measurement of glucose in aqueous solutions are shown in Fig. 4. We obtained an excellent linear response with correlation coefficient of 0.998 across the range of 100 mg/dL to 1.5 g/dL.
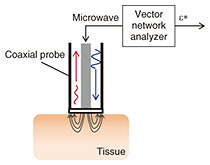
3. DSMicrowave glucose sensing has advantages over the use of optical sensors due to its lower energy per photon and smaller scattering from tissue. Two approaches to capturing the glucose footprint have been investigated. One involves measuring the frequency shift of the resonance in an LC (inductor-capacitor) resonator that corresponds to the change in permittivity at a specific frequency. The other involves measuring the complex permittivity over a wide frequency range by using the conventional methodology [6]. The former approach generally provides higher sensitivity to glucose thanks to its utilization of a high-Q (quality factor) resonator. However, it lacks selectivity to glucose. The effects of other components need to be compensated for in vivo and under practical conditions. With blood components, large molecules such as proteins may degrade accuracy because they exclude larger volumes of water that are dominant components in the dielectric properties. We focus on the spectroscopy-based sensors that can potentially provide high selectivity using preprocessing based on spectroscopic analysis. The experimental setup for carrying out DS of aqueous solutions using a coaxial probe and vector network analyzer is illustrated in Fig. 5. A one-port scattering parameter is measured, and the dielectric constant is calculated using measured data of calibration standards, air, electrical short circuits, and pure water.
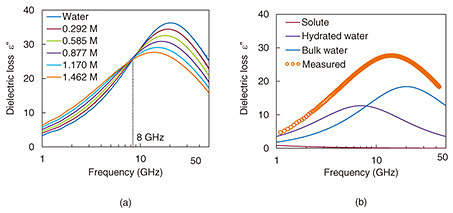
The imaginary parts of measured broadband complex dielectric spectra for different glucose concentrations (0–1.462 M) are shown in Fig. 6(a). Microwave DS is a potential tool for investigating the hydration dynamics of aqueous solutions to reveal the functionality of biomolecular systems. A comparison of the measured data versus the fitted curve based on a linear combination of Debye’s relaxation model is shown in Fig. 6(b) [7]. As the glucose concentration increases, the glucose-hydration water absorption at around 12 GHz increases. In contrast, bulk water absorption at around 20 GHz decreases. As a result, the peak of the dielectric spectrum shifts to a lower frequency and is broader than that of pure water.
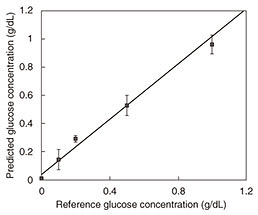
The results of in vitro measurement of glucose in aqueous solutions using DS in the frequency range of 500 MHz to 50 GHz are presented in Fig. 7. We obtained a linear response with a linear regression correlation coefficient of 0.987 over the range of 50 mg/dL to 1.0 g/dL. One difficulty with taking measurements on a living body is signal drift due to changes in temperature, water content, and other factors. We proposed a drift correction technique that has been standardized using the dielectric constant at 8 GHz [8]. Since the frequency point is the isosbestic point of the aqueous solution of glucose, the effect of water content on measurement is expected to be suppressed. We conducted in vivo measurements using only two frequency points in view of the expected future miniaturization of the system.
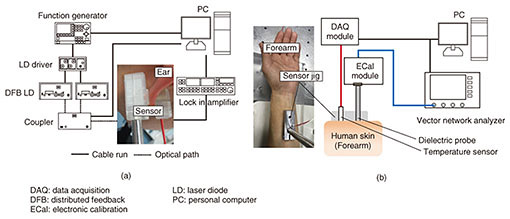
4. in vivo measurement and resultsEthical approval was obtained from the ethics committee at the University of Tokyo for in vivo measurements. All experiments were performed in accordance with relevant guidelines and regulations. Volunteers were healthy males and females aged from 21 to 31, and informed consent was obtained. We conducted oral glucose tolerance tests (OGTTs) to compare the results of PAS and DS with commercially available invasive glucose sensors. The glucose level during the OGTTs was monitored with a commercial FGM (FreeStyle Libre, Abbott Co.) [9]. A needle sensor was inserted into tissue under the skin of the left upper arm, and the glucose level in tissue was measured every five minutes. The experimental setup of the noninvasive glucose monitor is shown in Fig. 8 [8, 10]. The sensor interfaces of the PAS and DS were attached to the left earlobe (Fig. 8(a)) and forearm (Fig. 8(b)), respectively. For the OGTTs, liquid glucose containing 75 g of glucose (TRELAN G75, AY PHARMACEUTICALS CO., LTD.) was taken orally by the volunteers 30 minutes after the start of the measurement of PAS and DS to determine the base line. The signals were collected every minute, and the total duration of the test was three hours.
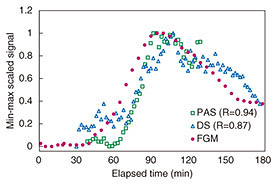
The temperature of the sample is uncontrollable in in vivo measurement, and the effect of tissue temperature on the measurement results is unknown. Temperature sensors were therefore integrated with each system, and the measured signals were corrected using the temperature data. The results of min-max scaling of PAS, DS, and FGM signals are plotted in Fig. 9. Our proposed measurement techniques show good traceability to the FGM. The respective correlation coefficients of PAS and DS were 0.94 and 0.84. The signals of noninvasive measurement appeared to be delayed by 10–20 min compared with FGM. We considered that this delay was caused by a difference in the penetration depth. FGM generally has a 10–15 min time lag in showing changes in the glucose level because of the time taken for blood components to travel from blood vessels to interstitial fluid. The time lag seen in Fig. 9 is thought to be due to the traveling time. However, the specific cause of this phenomenon is not clear. We will continue researching this to elucidate a measurement mechanism.
5. ConclusionWe introduced a means of noninvasive glucose measurement using PAS and DS. The measurement techniques were proposed to suppress the instability from biomedical samples, including the human body itself, for example, water absorption and signal drift during measurement. The results of in vivo measurement showed a high correlation between both techniques and the FGM. This result indicates the feasibility of using a noninvasive glucose sensor to visualize changes in glucose level. In the future, we plan to work on developing a calculation algorithm to determine absolute values and to miniaturize the system to a size small enough to be used as a wearable device. AcknowledgmentWe thank Dr. Kayo Waki and Ms. Ritsuko Nagatomo of the University of Tokyo for their help in conducting the in vivo measurements and for their valuable advice on glucose metabolism and glucose monitoring equipment. References
|
|||||||||||||||||||||