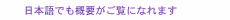|
|||||||||||||
|
|
|||||||||||||
|
Feature Articles: NTT’s Medical and Health Vision toward Creation of Bio-digital Twin Vol. 19, No. 7, pp. 34–39, July 2021. https://doi.org/10.53829/ntr202107fa4 Technology for Visualizing the Circadian Rhythm: Wearable Core-body-temperature SensorAbstractCore body temperature (CBT) is an important vital sign and has been attracting attention as an index reflecting the circadian rhythm of the human body. Since CBT is the temperature at the core of the body, it is necessary to insert a sensor into a body cavity to accurately measure it, which puts a heavy burden on the individual having his/her temperature taken. At NTT, focusing on the flow of heat in the body, we are researching technology that makes it possible to measure CBT simply by affixing a sensor to the body. In this article, the current progress of this research is introduced. Keywords: circadian rhythm, core body temperature, non-invasive 1. Advanced health management enabled by measurement of core body temperatureAs shown in Fig. 1(a), core body temperature (CBT) refers to the temperature of the core of the body, which includes the brain and organs. To protect the functions of those organs, CBT is not easily affected by the outside environment and is maintained at the highest temperature in the whole body. CBT increases as a result of inflammatory reactions, such as heat stroke and infectious diseases, and decreases with the onset of hypothermia and hypothermia treatment; therefore, it is used as an important vital sign. CBT fluctuates by about 1°C in a daily cycle even within the normal temperature range. This fluctuation is linked to the circadian rhythm of the body [1]. Recent studies have shown that circadian rhythms are closely related to our physical condition in terms of sleep, exercise quality, onset of illness, and so on. In today’s society, in which people have diversified lifestyles, it is easy for the circadian rhythm to become out of sync with the time of day. A common example is a person’s bedtime and time of getting up becoming out of sync with the time of day, which is related to various diseases that have become social problems. To understand the circadian rhythm of a person’s body, a common method is to collect blood and examine temporal changes in hormone components. Measuring CBT is also an effective method. As shown in Fig. 1(b), if there is no shift between the body’s rhythm and time of day, CBT begins to decrease a few hours before sleep and begins to increase in the second half of the sleep cycle. However, if the body’s circadian rhythm and the timing of sleep and waking up shift due to irregularities, the quality of sleep will deteriorate. Such a state is called “social jet lag,” since the person feels like they have jet lag. If this state continues, it will lead to sleep disorders, such as trouble sleeping, light sleep, difficulty getting up in the morning, and sleepiness during the day, which adversely affect physical and mental health as well as social activities [2]. The circadian rhythm can be improved by external stimuli such as light irradiation. Therefore, if it were possible to measure CBT without burdening the individual and easily understand a person’s circadian rhythm, as shown in Fig. 1(c), it would be possible to build a healthcare system tailored to each person. Accordingly, measuring CBT should contribute to various scenarios related to circadian rhythms, such as sleep management, nursing care, and labor management, as shown in Fig. 1(d).
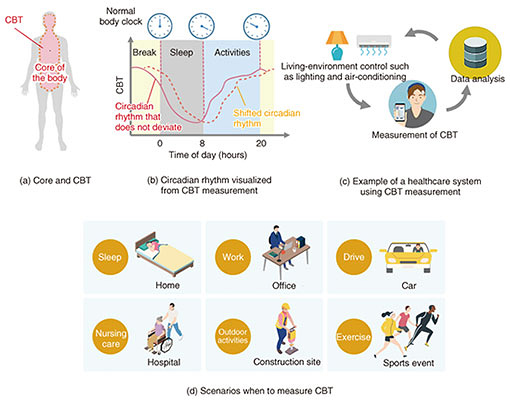
NTT has been developing highly accurate, low-burden CBT sensors for detecting slight variations in CBT (i.e., about 1°C in a day). In this article, we introduce current methods of measuring CBT, the principle of our non-invasive sensor for measuring CBT, and the current progress in our research. 2. Current methods for measuring CBT and challengesMethods for measuring CBT can be divided into two categories on the basis of their degree of invasiveness. The first category includes methods of inserting a thermometer into a body cavity and measuring the temperature. A sublingual thermometer is used to measure CBT by inserting it under the tongue and keeping the mouth closed for a certain period so that the measurement is not affected by breathing or eating. An eardrum thermometer is used to measure the eardrum temperature by measuring the infrared rays emitted from the eardrum with a sensor inserted into the ear. Since the temperature of the eardrum reflects the temperature of the nearby carotid artery, it is possible to measure CBT. During surgery, for strict control of CBT, rectal temperature is measured via an inserted sensor, and pulmonary-artery temperature is measured using an inserted catheter. Since these measurement methods are invasive, the thermometer is not easily affected by the external environment, and the measured temperature is reliable. Since the thermometer is inserted into the body, however, it places a heavy burden on the patient and requires caution in terms of hygiene. The other category includes methods of measuring temperature through contact with the surface of the body. Although it is hygienic, the thermometer comes into contact with the external environment, so the surface at which temperature can be measured is limited. An axillary thermometer is used to measure a temperature reflecting the CBT by closing the armpit tightly around the thermometer and keeping it there for about five minutes [3]. However, it is difficult to maintain this state for a long time. Therefore, a sensor using a method called the zero heat flux method (ZHFM) can be used to measure CBT even if the thermometer is attached to the body and exposed to the external environment. With this method, a sensor is affixed to the forehead, and CBT is measured by applying heat (via a heater inside the sensor) to cancel the heat flow (heat flux) from inside to the surface of the body. The sensor used with ZHFM requires a relatively large amount of electric power because it involves heating a living body. In addition, the environment in which it can be used is limited, so it is used to control CBT only during surgery. In light of these circumstances, as a method with low power consumption and few restrictions on the usage environment, and a sensor that measures the heat flux by being affixed to the surface of the body and estimates CBT without heating is needed. However, measurement errors likely occur with sensors that measure heat flux due to changes in the external environment (such as airflow variation) and changes in the person’s sweating. Accordingly, NTT is attempting to solve this problem by using a non-invasive method of measuring CBT, which is less burdensome for the individual having his/her temperature taken, involving affixing a sensor to the surface of the body. 3. Non-invasive measurement of CBT and features of our sensorThe non-invasive method for measuring CBT simply by affixing a sensor on the surface of the body (skin) is outlined in Fig. 2. CBT is manifested as skin temperature from the CBT region in the core of the body through biological tissues. Compared with CBT, the skin temperature is lower under normal, comfortable conditions owing to the dissipation of heat to the outside air. To convert skin temperature measured on the surface of the body to CBT, it is necessary to determine the temperature distribution from the region of the body corresponding to CBT to the surface of the body where the CBT sensor is affixed. In other words, the true CBT is estimated by calculating how much the skin temperature has dropped from the CBT. This is called the heat-flux method [4]. In previous research examples of a CBT sensor using the heat-flux method, as shown in Fig. 2(a), CBT could be accurately estimated in an experimental environment without air flow.
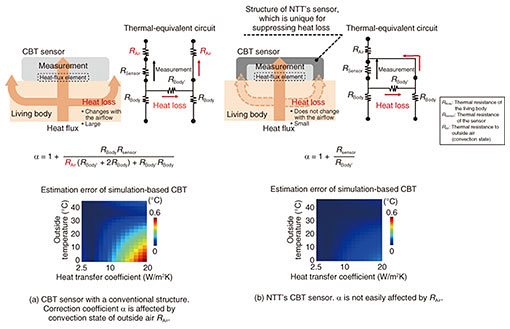
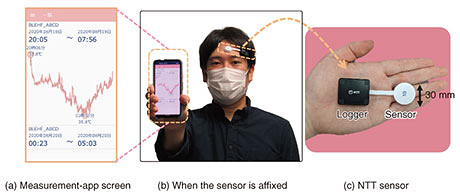
In environments where people live, there is rarely no airflow, so convection always exists. When a CBT sensor is affixed to the surface of the body, the skin temperature in the area covered by the sensor is higher than that around the sensor (which is not covered). This state causes heat to flow outwards from the sensor, as shown with the arrows in Fig. 2(a). This heat loss is heat that cannot be measured in the sensing area, thus causing errors in CBT estimation, as shown in Fig. 2(a). One method of estimating CBT is calibrating a correction factor (α) in advance as the ratio of heat flux (measured using the heat-flux sensor) to heat loss [5]. However, α is also affected by changes in convective conditions, which also cause estimation errors. With such estimation errors in mind, we developed a sensor for which α is less sensitive to convective conditions [6]. The structure of this sensor is outlined in Fig. 2(b). In a conventional CBT sensor, the leaked heat flux is directly dissipated to the outside air without going through the sensor, and α depends on the convection state. Our sensor is surrounded with a high-heat-conduction material that allows heat to pass through easily, and the leaked heat flux passes through the high-heat-conduction material, merges with the heat flux measured with the heat-flux sensor, and dissipates to the outside air. Therefore, α is independent of the convective state, and errors in CBT estimation can be reduced. The structure of this sensor is called the heat-loss-suppression structure. By optimizing the shape of this structure and minimizing heat loss, our sensor is smaller and is more accurate than a conventional CBT sensor. As shown in Fig. 2(b), using this optimized sensor makes it possible to suppress errors in CBT estimation to within 0.1°C, even when the airflow rate is 5 m/s, which is stronger than the airflow rate of an air conditioner. 4. Example results of measuring CBT by using our developed sensorOur CBT sensor with the heat-loss-suppression structure is shown in Fig. 3. The sensor part (white probe) has a diameter of 30 mm, which is about the same size as a plastic-bottle cap, and a thickness of 5 mm, which is one-third that of the cap. Skin temperature and heat flux measured with the sensor are digitally converted by the logger, transferred to the measurement app via Bluetooth, and recorded.
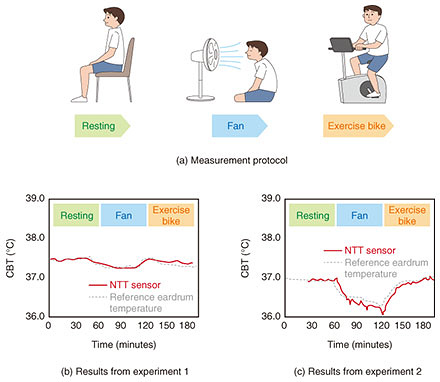
Measurement results from an experiment in which the CBT sensor was affixed to a person’s body is shown in Fig. 4. The sensor was affixed to the forehead of two different participants in two experiments: one in which air was blown by a fan at the participant, and one in which the participant performed low-intensity exercise (on an exercise bike). In both experiments, the reference temperature (of a commercially available eardrum sensor) highly correlated with the temperature measured using our senor. These results indicate that CBT can be accurately measured with this sensor even in an environment in which convection exists, such as in a room cooled with an air conditioner.
5. Future prospectsIn this article focusing on implementation of a non-invasive CBT sensor that can be simply affixed to the surface of the body, we introduced a method for estimating CBT from heat flux. If daily high-precision CBT measurement is possible, which had been difficult, it can be used for health-management systems and new medical treatments such as chronopharmacology. If it becomes possible to visualize the body’s circadian rhythms from fluctuations in CBT and understand deviations from daily rhythms, it will be possible to implement applications that maintain the body’s circadian rhythms appropriately by controlling the environment in cooperation with smart homes and other devices. For long-term daily monitoring, usability, such as wearability and continuous measurability, as well as connection with smartphones for data collection and visualization are key factors. We will continue research and development on long-term continuous measurement using a device that integrates a sensor and logger. References
|
|||||||||||||