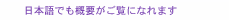|
|||||||||||||
|
|
|||||||||||||
|
Regular Articles Vol. 23, No. 5, pp. 42–48, May 2025. https://doi.org/10.53829/ntr202505ra1 Proposal of a Transdermal Iontophoresis Patch and Sheet Mask Using the Principle of Magnesium-air BatteriesAbstractTo develop an iontophoresis technology that does not require a large power supply, this study focused on magnesium-air batteries, which use atmospheric oxygen and magnesium, a metal with a low incidence of allergic reactions. We thus propose a transdermal iontophoresis patch and sheet mask using the principle of magnesium-air batteries. To verify the feasibility of the transdermal iontophoresis patch and sheet mask, we conducted an evaluation using time-of-flight secondary ion mass spectrometry. Ion mapping of the active ingredient and analysis of its line profile revealed that the use of our iontophoresis patches enhanced active-ingredient penetration by approximately twice that of patches without iontophoresis. These findings indicate that the patch and sheet mask technologies contribute to the development of a highly portable and user-friendly transdermal iontophoresis patch and sheet mask that can be easily used at home. Keywords: iontophoresis, magnesium-air batteries, transdermal delivery 1. Transdermal-absorption-enhancement technologyThe medication method through which a patch to the skin surface (transdermal drug delivery) is applied has been attracting attention as the third route of medication, following oral and injectable methods. Transdermal drug delivery offers various advantages compared with oral administration [1]. It is suitable for drugs that undergo significant first-pass effect in the liver, as transdermal administration can bypass early metabolism. Transdermal delivery also has benefits over subcutaneous injections. For example, subcutaneous injections can be painful, whereas transdermal administration is non-invasive and allows for self-administration, contributing to improved patient compliance. Transdermal absorption has a history of only about 40 years [2], and the market size is expected to continue expanding. Beyond the medical field, transdermal-absorption technology is also being integrated into the cosmetics field. Skincare products, such as sheet masks, which incorporate active ingredients into lotions and serums for skin absorption, are becoming an established part of skincare routines. However, transdermal drug delivery faces limitations due to the skin barrier, which restricts the amount of drug that can be absorbed and limits the range of applicable drugs. To address this challenge, iontophoresis—a technology that uses weak electrical currents to enhance the penetration of active ingredients through the skin—has been under research for many years. While most of these technologies require large power-supply devices and are not as convenient as patches or sheet masks, research has focused on the development of disposable, easy-to-use iontophoresis patches, garnering significant attention [3, 4]. 2. Iontophoresis patches and sheet masks using the principle of magnesium-air batteriesBy focusing on magnesium-air batteries, which use atmospheric oxygen and magnesium (a metal with a low incidence of allergic reactions), we propose a transdermal iontophoresis patch and sheet-mask mock-up using the principle of magnesium-air batteries, as shown in Figs. 1 and 2, respectively.
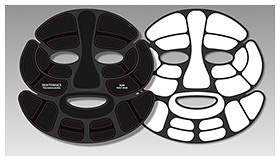
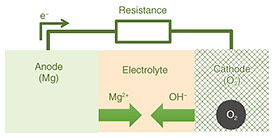
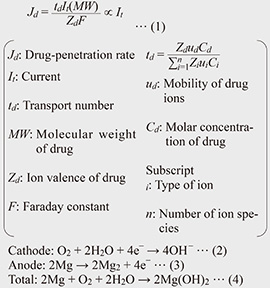
2.1 Principle and patch structureFigure 3 illustrates the structure of a magnesium-air battery and its electrochemical reactions. A magnesium-air battery consists of an air electrode, which serves as the reaction site for oxygen as the cathode active material, a magnesium anode, and an electrolyte that facilitates ion transport. The fundamental structure of the proposed iontophoresis patches is based on replacing the electrolyte of the magnesium-air battery with the skin (Fig. 4).
During battery reaction, ions flow from both the cathode and anode into the skin, and the resulting electro-osmosis is expected to enhance the penetration of active ingredients. The penetration rate of active ingredients in iontophoresis is given by Eq. (1) [5] and is proportional to the applied current. Therefore, increasing the penetration amount of active ingredients requires enhancing the output power of the magnesium-air battery. To achieve higher power in a magnesium-air battery, it is necessary to promote the reactions described in Eqs. (2) and (3). However, since the cathode reaction often acts as the rate-limiting step, a key challenge is how to activate and stabilize the cathode reaction effectively.
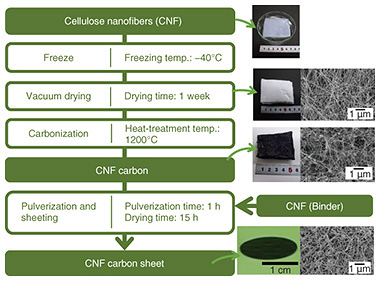
2.2 Fabrication method of cellulose nanofiber carbon sheetAs in Eq. (2), the cathode reaction becomes more active when oxygen (gas), water (liquid), and electrons (in the carbon solid) coexist. To satisfy this condition, it is necessary to have a greater number of reaction sites where oxygen, electrolyte, and carbon coexist—known as three-phase interfaces. We focused on cellulose nanofiber (CNF) carbon, in which carbon nanofibers form a mesh-like structure, to increase the number of three-phase interfaces. The fabrication process of a CNF carbon sheet is shown in Fig. 5. Cellulose nanofibers were rapidly frozen at −40°C, followed by vacuum drying for more than 1 week. Subsequently, heat treatment was conducted at 1200°C for 1 hour under a nitrogen atmosphere to obtain CNF carbon. The fabricated CNF carbon was then mixed and milled with CNF, filtered by suction, and dried to form a sheet.
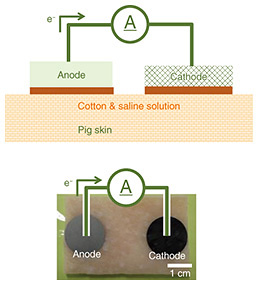
Figure 5 shows photographs and scanning electron microscope (SEM) images of the fabricated samples. The SEM images indicate that the mesh structure of the carbon was maintained both after carbonization of the CNF and after sheet formation. 3. Evaluation of current and permeation test of proposed iontophoresis patch3.1 Evaluation of currentFigure 6 shows a schematic diagram and photograph of the setup of evaluating current using pig skin. The pig skin used was 3 × 4 cm, and the electrodes had diameters of 16 mm each, with a center-to-center distance of 3 cm. A cotton pad was placed under the electrodes, onto which 300 μl of saline solution was applied.
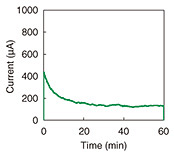
Figure 7 shows the results of the current measurement. A current of over 200 μA was obtained, indicating that the output is sufficient for evaluating iontophoresis.
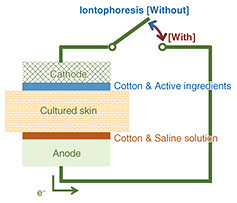
3.2 Permeation testFigure 8 illustrates the setup for the permeation test using cultured skin. The cultured skin used was the three-dimensional artificial cultured skin “LabCyte EPI-MODEL12” from Japan Tissue Engineering Co., Ltd. The active ingredients used were saturated aqueous solutions of niacinamide and tranexamic acid, provided by Tokyo Chemical Industry Co., Ltd. The setup consisted of layers arranged in the following order: aluminum (conductive layer)/CNF carbon electrode/cotton/cultured skin/cotton/magnesium electrode/aluminum (conductive layer).
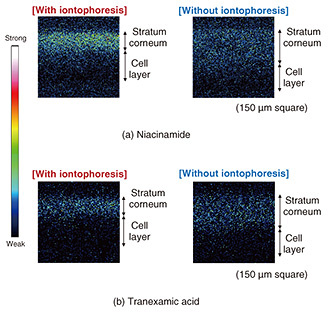
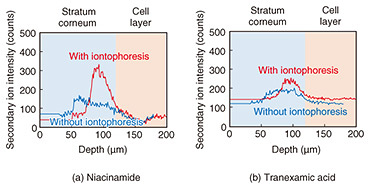
A saline solution was applied to the cotton on the magnesium-electrode side, while the prepared active ingredient solution was applied to the CNF carbon electrode. The setup was then sandwiched between glass slides and left stationary for one hour. The conductive layer, electrodes, and cotton were then gently removed, and the cultured skin was frozen at −18°C using a cryostat. The skin was embedded in a compound resin, sectioned into 10 μm-thick slices, and analyzed using time-of-flight secondary ion mass spectrometry (TOF-SIMS) to obtain ion image mapping of the cross-section of the cultured skin. TOF-SIMS analysis was used to obtain data on PC5H15NO4 (m/z = 184.1) derived from phospholipids, a marker derived from the cell layer, to distinguish the stratum corneum from the cellular layer in the cultured skin cross-section. Figure 9 shows TOF-SIMS ion images of the cross-section for each active ingredient, while Figure 10 shows the TOF-SIMS line profile. A significant difference in approximately twice that of patches without iontophoresis was observed in the penetration of niacinamide with iontophoresis. In contrast, no significant difference was observed for tranexamic acid. This is attributed to the difference in their ionic properties; niacinamide is cationic with a pKa (acidity constant) of 3.3, while tranexamic acid is amphoteric with a pKa1 of 4.33 and pKa2 of 10.65, leading to differences in penetration rates.
4. Future prospectsThis study suggests the feasibility of portable iontophoresis patches and sheet masks that can be easily used at home. And these transdermal iontophoresis patches and sheet masks using the principle of magnesium-air batteries would enable remote drug administration, eliminating the need for hospital visits for injections. This would be particularly beneficial for young children, who could avoid the pain and anxiety associated with injections. For those who do not have the strength to go to the hospital, this approach would allow for drug administration anytime and anywhere with minimal effort. In case of adverse effects, the patch could be promptly removed. In regions with shortages of healthcare professionals or in developing countries with limited medical infrastructure, our patch and sheet mask could facilitate mass vaccination before an epidemic outbreak and reduce waiting times for vaccinations. Integrating our patch and sheet mask with real-time biometric monitoring systems could lead to personalized drug administration linked to physiological data, such as blood-glucose levels. By incorporating artificial-intelligence analysis and predictive modeling, a closed-loop system could be established, enabling real-time adjustment of drug administration based on feedback. This could ultimately contribute to a Bio-digital Twin™*, with which biological, behavioral, and medical health information is continuously collected, analyzed, and used for predictive disease management and optimized treatment.
References
|
|||||||||||||